Matthew Whorton
B.S. Duke University
Ph.D. University of Michigan
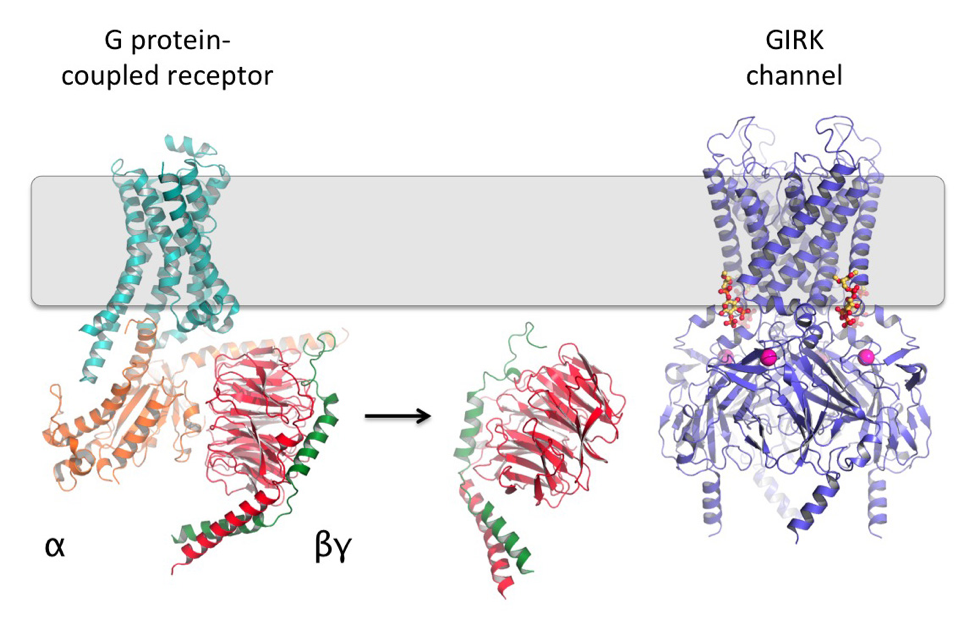
Many hormones and neurotransmitters work by binding to G protein-coupled receptors (GPCRs) to activate intracellular G proteins, which then have a multitude of effects on a cell. One well-established role for G proteins is the regulation of ion channel conduction. This is one way hormones and neurotransmitters can control cellular excitability. A classic example of such a system is the activation of G protein-gated inward rectifier K+ (GIRK) channels by the βγ subunits of G proteins (Gβγ). This causes cells to hyperpolarize, which underlies many physiological processes such as the control of neuronal activity and the beating rate of the heart. GIRK channels are also activated by PIP2 and intracellular Na+ ions, and I have been interested in how all of these ligands work in concert to regulate GIRK gating.
To address this, I have determined several X-ray crystal structures of GIRK, which revealed the binding sites for PIP2 (red and yellow molecules above) and Na+ (purple spheres below). Based on these structures, I tested the function of many point mutants in electrophysiological assays and discovered a constitutively active mutant. I then determined crystal structures of this mutant in the absence and presence of PIP2, which revealed conformational changes that are probably similar to what Gβγ-binding would induce. On-going biochemical and crystallographic studies aim to further elucidate the GIRK gating mechanism.
Reference: Whorton & MacKinnon, Cell, 2011.
