Current PND Research
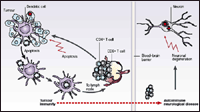
We are currently actively interested in studying the pathophysiology of the PNDs, and the clinical implications of the disorders. These include clinical research studies (ADO-0497; RDA-0148; RDA-0039), in which we are assessing the nature of the immune responses in PND patients, and, where appropriate, trials of new treatments for the PNDs. From our studies, we have learned that patients with PCD harbor antigen (cdr2)-specific killer T cells ( Albert ML, Darnell JC, Bender A, Francisco L, Bhardwaj N and Darnell RB. Nature Med, 4:1321-1324, 1998 and Albert ML, Austin LM and Darnell RB. Ann Neurol, 47:9-17, 2000), in addition to cdr2-specific antibodies.
We have studied the mechanism by which cdr2-specific killer T cells initially become activated. Such activation entails a paradox ( Albert ML and Darnell RB. Nature Rev Cancer, 4:36-44, 2004), as classical immunologic thinking teaches that such activation would occur in lymph nodes by antigen presenting cells expressing endogenous cdr2 protein, yet expression analysis indicated that cdr2 expression is restricted to brain and testis. This paradox, and observation by Antony Rosen and colleagues that autoantigens were "packaged" in membrane-bound blebs after induction of apoptotic death, led us to hypothesize that apoptotic tumor cells might provide a source of antigen that could be transferred to APCs to initiate PND tumor immune responses ( Darnell RB. Proc Natl Acad Sci, USA, 93:4529-4536, 1996).
This prediction was tested in PCD by feeding apoptotic tumor cells expressing the cdr2 antigen to autologous patient dendritic cells (DCs; potent APCs discovered by Steinman and colleagues at The Rockefeller University. We found that such apoptotic tumor-fed DCs were extremely potent at stimulating patient killer T cells ( Albert ML, Darnell JC, Bender A, Francisco L, Bhardwaj N and Darnell RB. Nature Med, 4:1321-1324, 1998 and Albert ML, Austin LM and Darnell RB. Ann Neurol, 47:9-17, 2000. Similar findings were made using apoptotic virus infected cells by our colleagues Matthew Albert and Nina Bhardwaj ( Albert ML, Sauter B, Bhardwaj N. Nature. 1998 Mar 5;392(6671):86-9).
These observations have led us to a revised model for the pathogenesis of the PNDs. Ongoing work in the laboratory is aimed at refining this model. Efforts are aimed both at understanding why some, but not all patients harboring tumors expressing PND antigens generate effective tumor immune responses, and how such tumor immune responses in the body cross into the brain to generate autoimmune brain disease.
Our studies of PND pathogenesis have taken us back to the clinic, in efforts to try and recapitulate the potent tumor immune responses present in PND patients in the general population of cancer patients. We have found that for DCs require a signal from CD4+ T cells to cross-present apoptotic antigen and activate (rather than induce tolerance in) CD8 T cells ( Albert ML, Jegathesan M, Darnell RB. Nat Immunol. 2001 Nov;2(11): 1010-7). We have also found that apoptotic cells may process antigen prior to delivery to DCs ( Blachere NE, Darnell RB, Albert ML. PLoS Biol 2005 Jun; 3(6):e185. Epub 2005 Apr 26), an observation which may be of importance in understanding tumor immunology and autoimmunity. These studies have led to clinical interventional studies (RDA-0466; RDA-0508) in which new apoptotic- tumor based vaccines are being developed for the general population of cancer patients. We continue to study the necessary and sufficient components for T cell activation by cross-presentation of apoptotic tumor cells.
