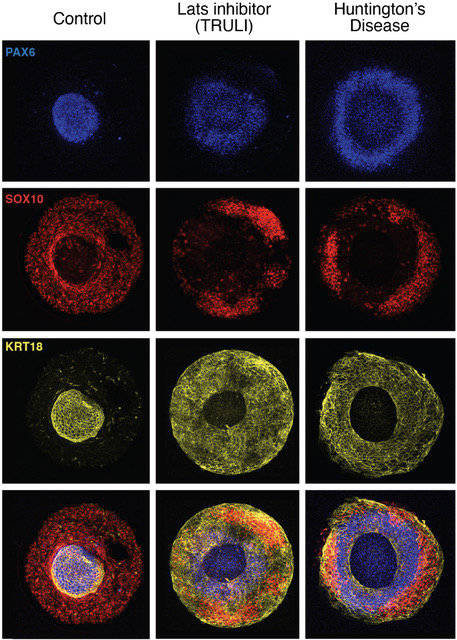
Role of Yap in early ectodermal specification and a Huntington's Disease model
The Hippo pathway, a highly conserved signaling cascade that functions as an integrator of molecular signals and biophysical states, ultimately impinges upon the transcriptional coactivator Yap. Hippo-Yap signaling has been shown to play key roles both at the early embryonic stages of implantation and gastrulation, and later during neurogenesis. To explore Yap's potential role in neurulation, we used self-organizing neuruloids grown by the Brivanlou group from human embryonic stem cells on micropatterned substrates. By using a selective inhibitor of Lats kinases, we identified Yap activation as a key lineage determinant, first between neuronal ectoderm and non-neuronal ectoderm, and later between epidermis and neural crest. Because aberrant Hippo-Yap signaling has been implicated in the pathology of Huntington’s Disease, we used isogenic mutant neuruloids to explore the relationship between signaling and the disease. We found that Huntington’s Disease neuruloids demonstrate ectopic activation of gene targets of Yap and that pharmacological reduction of Yap's transcriptional activity can partially rescue the Huntington’s Disease phenotype.