Semaphorin 7A patterns neural circuitry in the lateral line of the zebrafish
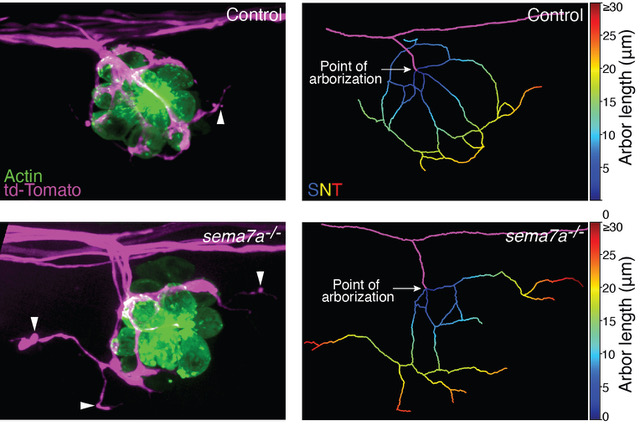
In a developing nervous system, axonal arbors often undergo complex rearrangements before neural circuits attain their final innervation topology. Developing sensory axons of the zebrafish's lateral-line sensory system reorganize their terminal arborization patterns to establish precise neural microcircuits around the mechanosensory hair cells. However, we lack a quantitative understanding of the changes in the sensory arbor morphology and the regulators behind the microcircuit assembly. We have determined that semaphorin 7A acts as an important mediator of these processes. Utilizing a semi-automated three-dimensional method for tracing neurites, we have identified and quantitatively analyzed the topological features that shape the network in wild-type and semaphorin 7A loss-of-function mutants. In contrast to those of wild-type animals, the sensory axons in sema7A‑/‑ mutants display aberrant arborizations with disorganized network topology and diminished contacts with hair cells. Moreover, ectopic expression of a secreted form of semaphorin 7A by non-hair cells induces chemotropic guidance of sensory axons. Our findings demonstrate that semaphorin 7A functions both as a juxtracrine and as a secreted cue to pattern neural circuitry during the development of a sensory organ.