Phytochrome A
The Arabidopsis phytochrome family comprises five photoreceptors that exist in two photointerconvertible forms: a red light-absorbing Pr form and a far-red light-absorbing form Pfr. Two important features distinguish phytochrome A from the other four phytochromes in Arabidopsis:
- whereas phyB through phyE are activated exclusively by red light and inactivated by subsequent irradiation with far-red light, phyA can be activated by far-red light as well as low fluences of red and blue light;
- whereas phyA is by far the most abundant phytochrome in dark-grown seedlings, exposure to red light decreases its half-life from approximately 100 h (in etiolated seedlings) to less than 1 h. The degradation of phyA coincides with a transient accumulation of ubiquitin-Pfr conjugates.
The extreme photolability of phyA exemplifies the importance of regulated proteolysis in feedback inhibition of signal transduction cascades. The light-regulated differential degradation of phyA enables germinating seedlings to be exquisitely sensitive to light until the apical hook emerges from the soil surface. Thereafter, the pathway is desensitized to ensure allocation of resources to photomorphogenic development.
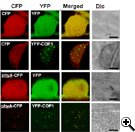 Reproduced, with permission, from Genes and Development Vol 18 (6). © 2004 by Cold Spring Harbor Laboratory Press.
Reproduced, with permission, from Genes and Development Vol 18 (6). © 2004 by Cold Spring Harbor Laboratory Press.Like other phytochromes, irradiation of etiolated plants triggers the translocation of phyA from the cytosol to discrete sites in the nucleus. This translocation appears to be activated by light wavelengths and fluences specifically associated with phyA-triggered responses. In the nucleus, phyA initiates a signaling cascade that alters the expression of light-regulated genes. The significance of the transient accumulation of the translocated phyA in nuclear bodies remains enigmatic, although it is intriguing that COP1 also aggregates in nuclear bodies in etiolated seedlings. The co-localization of the ubiquitin ligase COP1 and its substrate LAF1 in the same nuclear aggregates led us to propose that these nuclear bodies may be associated with proteolysis. Indeed, we have subsequently shown that the phyA PAS domain interacts with the WD40 domain of COP1 and that the rate of phyA destruction in irradiated seedlings is decreased in cop1 mutants and by expression of a COP1 RING motif mutant protein. These findings indicate that COP1 acts as an E3 ligase to regulate phyA signaling by targeting elimination of the photoreceptor itself.
