Role of Fibrin(ogen) in Alzheimer's Disease
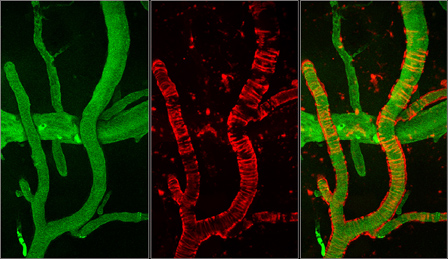 |
The images were taken using a multiphoton microscope and illustrate the vasculature of an Alzheimer's disease mouse. Green shows blood flow and red amyloid deposition. The ring-like structures surrounding the blood vessels represent cerebral amyloid angiopathy. |
Alzheimer’s disease (AD) is a neurodegenerative disorder that leads to memory loss and death. The β-amyloid peptide (Aβ) has been implicated in AD by analysis of genetic mutations linked to early onset cases of the disease. However, the mechanism of how Aβ contributes to cognitive decline is unknown. Much evidence suggests that AD has a vascular component. Patients present Aβ deposits in the vessels, known as cerebral amyloid angiopathy (CAA), which provokes the degeneration of vessel wall components and affects cerebral blood flow. In addition, epidemiology links vascular diseases with an increased risk for dementia and AD, and AD patients suffer from cerebrovascular dysfunction and decreased cerebral blood flow. Thus, circulatory deficiencies could play an important role in AD pathogenesis, with neuronal loss and memory deficits caused by vascular problems.
Elevated fibrinogen levels are correlated with increased risk for AD, and fibrinogen accumulates in the extravascular space in this disease. Previous studies from the lab reported that fibrin(ogen) deposition potentiates the increase in blood brain barrier permeability and neurovascular damage seen in AD mice. Now we are interested in analyzing in detail the relationship between Aβ and fibrinogen in AD. We have performed in vitro and in vivo experiments that showed that fibrin clots formed in the presence of Aβ are structurally abnormal and resistant to degradation. Also, fibrin(ogen) was observed in blood vessels positive for amyloid in mouse and human AD samples, and intravital brain imaging of clot formation and dissolution revealed abnormal thrombosis and fibrinolysis in AD mice. Moreover, depletion of fibrinogen lessened cerebral amyloid angiopathy pathology and reduced cognitive impairment in AD mice.
Based on these data, we hypothesize that in the presence of Aβ any fibrin deposits formed would be more persistent, provoke aberrant hemostasis and lead to compromised blood flow, increased inflammation, and eventually cognitive decline. Our results suggest that one important contribution of Aβ to AD is via its effects on fibrin clots, implicating fibrin(ogen) as a potential critical factor in this disease. Therefore, exploring this association in detail will allow us to better understand the mechanisms of AD pathology and to identify potential therapies.
::: Back to Top ::: Back to List of Projects
