Nanomechanics of wild-type and mutant dimers of the tip-link protein protocadherin 15
Mechanical force controls the opening and closing of mechanosensitive ion channels atop the hair bundles of the inner ear. The filamentous tip link that connects each transduction channel to the tallest neighboring stereocilium modulates the force transmitted to the channel and thus changes its probability of opening. Each tip link comprises four molecules: a dimer of protocadherin 15 (PCDH15) and a dimer of cadherin 23, all of which are stabilized by Ca2+ binding. Using a high-speed optical trap to examine dimeric PCDH15, we find that the protein's mechanical properties are sensitive to Ca2+ and that the molecule exhibits limited unfolding at a physiological Ca2+ concentration. PCDH15 can therefore modulate its stiffness without undergoing large unfolding events under physiological conditions. The experimentally determined stiffness of PCDH15 accords with published values for the stiffness of the gating spring, the mechanical element that controls the opening of mechanotransduction channels. When PCDH15 exhibits a point mutation, V507D, associated with non-syndromic hearing loss, unfolding events occur more frequently under tension and refolding occurs more slowly than in the wild-type protein. Our results suggest that the maintenance of appropriate tension in the gating spring is critical to the appropriate transmission of force to transduction channels, and hence to hearing.
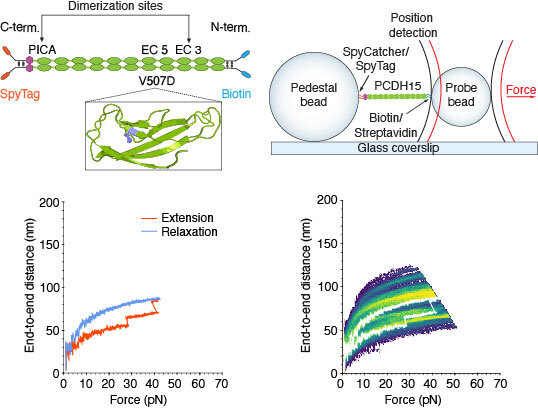
Top left: Extracellular cadherin domains are composed primarily of β barrels. Ca2+ binding in the linker regions and at the edges of the domains stabilizes the structure against unfolding. We maintain the dimerization sites in PCDH15 while adding two disulfide bonds and a distinct molecular tag at each end. The V507D construct is identical to the wild-type construct save for the insertion of a mutation (purple in inset). Top right: While a dimeric protein molecule is tethered between two beads, two laser beams act on the probe bead to measure its position and exert force on it. Bottom left: A force-ramp experiment comprises the 350 ms extension phase of the cycle during which force is increased at a constant rate, and the relaxation phase during which force is decreased back to a minimum in the same period. In these experiments, the minimum force is 1 pN and 2 s elapses between successive cycles. Unfolding events can be seen as sudden steps during the extension. Bottom right: Repetition of a force-ramp cycle hundreds of times on the same protein molecule yields a heatmap in which the brighter colors represent more highly occupied states. The heatmap superimposes both extension and relaxation phases for every cycle. The illustrative record from the bottom left panel is overlaid in white.
