Integrin Structure and Activation
Integrins are transmembrane glycoproteins involved in interactions between cells and between cells and the extracellular matrix. Integrins are heterodimers formed by an α and a β chain. Two highly homologous β3-containing integrins have been identified: αVβ3 and αIIbβ3. αVβ3 is present on the surface of numerous cell types (endothelial cells, smooth muscle cells, leukocytes, platelets, osteoclasts, mesangial cells), while αIIbβ3 is found only on platelets and their bone marrow precursors, the megakaryocytes.
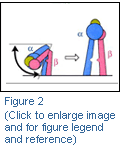
The solution, in 2001, of the crystal structure of αVβ3 (Figure 1) (Xiong et al., Science. 2001 Oct 12;294(5541):339-45) showed that the receptor is formed by a large globular head, the ligand binding domain, and two legs that enter the plasma membrane. Quite surprisingly the receptor appears to be in a bent conformation, with the ligand binding domain only partially accessible. Takagi and Springer have proposed a switchblade mechanism of integrin activation (Figure 2): after cell activation the receptor assumes an open position, allowing the ligand access to the binding region.
Through site-directed mutagenesis and molecular modeling studies of the integrin receptors αIIbβ3 and αVβ3, we are pursuing two main goals:
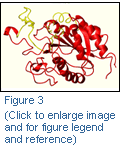
- identification of the 7E3 epitope on the β3 βA-domain (Figure 3)
- assessment of the switchblade activation model.