Laboratory of Genetics
Michael W. Young
Richard and Jeanne Fisher Professor
Biological Clocks
Mutations in several genes have strong effects on circadian (~24 hour) locomotor activity rhythms of Drosophila. These rhythms can be compared to human sleep/wake cycles. Indeed human orthologs of three of these Drosophila "clock" genes have been associated with disorders of sleep.
 In Drosophila, mutations of the period (per), timeless (tim), double-time (dbt), Clock (Clk), cycle (cyc), shaggy (sgg), vrille (vri) and Par-Domain Protein 1 (Pdp1) loci can lengthen or shorten the period of the locomotor activity rhythms, or can abolish the rhythms altogether. The abundance of per, tim, vri, Pdp 1 and Clk RNA and their encoded proteins changes rhythmically with a circadian period in wild-type flies. Mutations affecting any of the eight genes have corresponding effects on behavioral and molecular rhythms. Thus, molecular rhythms likely drive the behavioral rhythms.
In Drosophila, mutations of the period (per), timeless (tim), double-time (dbt), Clock (Clk), cycle (cyc), shaggy (sgg), vrille (vri) and Par-Domain Protein 1 (Pdp1) loci can lengthen or shorten the period of the locomotor activity rhythms, or can abolish the rhythms altogether. The abundance of per, tim, vri, Pdp 1 and Clk RNA and their encoded proteins changes rhythmically with a circadian period in wild-type flies. Mutations affecting any of the eight genes have corresponding effects on behavioral and molecular rhythms. Thus, molecular rhythms likely drive the behavioral rhythms.
The PER, DBT, TIM and SGG proteins physically interact. Interactions first occur in the cytoplasm. DBT, an ortholog of Casein Kinase 1, phosphorylates PER, triggering rapid PER degradation. PER's interaction with TIM blocks this DBT-dependent phosphorylation allowing accumulation of PER and formation of an "interval timer" that holds PER/TIM partners in the cytoplasm for several hours. Nuclear localization appears to be preceded by dissociation of PER/TIM complexes. TIM is eventually phosphorylated in a pathway that requires the kinase SGG, an ortholog of GSK-3, and this may contribute to nuclear localization of DBT, PER and TIM. In the nucleus, TIM-free PER strongly suppresses activity of two transcription factors encoded by Clk and cyc. This regulation is significant because in the absence of nuclear PER these transcription factors activate per and tim expression. As in the cytoplasm, TIM-free PER appears to be phosphorylated by DBT in the nucleus. Consequently transcription of per and tim resumes after an interval of nuclear PER phosphorylation and degradation. Two more cycling transcription factors, Vrille and PDP1, form a second feedback loop by regulating transcription of Clk. Vrille is a repressor of Clk and PDP1 is a Clk activator. Each affects Clk transcription at a different time of day, resulting in oscillating Clk expression.
TIM protein couples this molecular oscillator to the environment because TIM is rapidly degraded following exposure to light. Ordinarily cycles of light and dark restrict accumulation of PER-containing complexes to times after nightfall since TIM is degraded by light and TIM is required to stabilize PER. However, pulses of light occurring in the early evening or just prior to dawn reset the cycle by prematurely eliminating TIM in the cytoplasm or nucleus, respectively. In this way phase-shifts are induced in the behavioral rhythms. The acute-light sensitivity of TIM involves SGG, and function of an unusual photoreceptor, Cryptochrome (CRY).
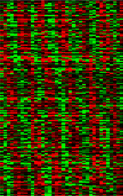 We have begun to look at programs of gene expression that are regulated by this molecular clock using oligonucleotide microarrays representing all genes (~14,000) of the fly. We find that in the Drosophila head, ~400-500 genes are expressed with a circadian rhythm (see data). This represents 6-7% of the genes that are active in the head. Genes composing this large circadian program influence almost every aspect of the fly's biology, and subsets of these genes are switched on and off with phases representing every hour of the day and night. Mutations of genes composing the clock appear to abolish this program of temporally sequenced gene expression, even when environmental cycles are provided. This indicates a thorough dependence of the temporal program on the identified molecular oscillator.
We have begun to look at programs of gene expression that are regulated by this molecular clock using oligonucleotide microarrays representing all genes (~14,000) of the fly. We find that in the Drosophila head, ~400-500 genes are expressed with a circadian rhythm (see data). This represents 6-7% of the genes that are active in the head. Genes composing this large circadian program influence almost every aspect of the fly's biology, and subsets of these genes are switched on and off with phases representing every hour of the day and night. Mutations of genes composing the clock appear to abolish this program of temporally sequenced gene expression, even when environmental cycles are provided. This indicates a thorough dependence of the temporal program on the identified molecular oscillator.
Recently, in collaboration with investigators at Weill-Cornell Medical College, we have been collecting human behavioral and molecular data from subjects with persistently delayed timing of the major sleep episode. In model animal systems, mutations that affect the period of circadian behavioral rhythms produce correlated effects that are evident at the level of oscillating gene activities in peripheral cells such as liver, muscle and skin. We are therefore analyzing dermal fibroblast cultures derived from such subjects for evidence of an altered circadian clock. Transfection of these cultured cells with circadian reporters has allowed a detailed characterization of the circadian rhythms of the subjects. The studies are providing strong evidence that molecular changes in the circadian clock can underlie delayed patterns of human sleep.
