WELCOME TO OUR IFAR FAMILY WEBSITE
Click on a header below to navigate directly there
- IFAR GENERAL INFORMATION
- FANCONI ANEMIA
- GENETICS OF FANCONI ANEMIA
- FOR FAMILIES ALREADY ENROLLED IN THE IFAR
- FOR FAMILIES NOT YET ENROLLED IN THE IFAR
- IFAR FORMS
- MEDICAL UPDATE FORM
- RESOURCES/LINKS
| IFAR GENERAL INFORMATION | FOR FAMILIES NOT YET ENROLLED IN THE IFAR |
| FANCONI ANEMIA | IFAR FORMS |
| GENETICS OF FANCONI ANEMIA | RESOURCES/LINKS |
| FOR FAMILIES ALREADY ENROLLED IN THE IFAR |
We asked. You answered. We listened...
Results of our recent study are available!
We are excited to update everyone about the results of our Patient Perspective Study. In this study, individuals enrolled in the IFAR were invited to participate in an online questionnaire through Survey Monkey to help us learn more about the motivations for participation, experiences with current services, and needs going forward. We thank everyone who participated in the survey for their time and ideas. We couldn't do this work without you and want to do everything possible to maximize your benefits while in the IFAR. Please click here to download our newsletter with all of these results.
IFAR stands for International Fanconi Anemia Registry. It is a research study that began at Rockefeller University in New York City in 1982. The purpose of the IFAR is to study the nature, diagnosis, and treatment of individuals with Fanconi anemia (FA). We hope that information collected in this study will help researchers better understand FA and be able to better diagnose and treat the condition, which can then directly benefit individuals and families affected by FA.
What can the IFAR offer?

Genetic counseling We offer genetic counseling to help answer questions you may have about the inheritance of FA, family members that may be at risk, and testing options available to you or your family. You can reach us at 212-327-8612 or fanconiregistry@rockefeller.edu.
Support We know what a hard journey this can be for families. We want to take this journey with you as much as we can and support you through it.
Knowledge While we recognize that you and your family have become the experts in FA, our team is made up researchers engrossed in the FA world. We are available to help answer questions you may have at any time.
Establish connections If you are not in touch with other FA families and would like to be, we can help you get connected. If you are looking for a physician/genetic counselor in your local area who is knowledgeable about FA, we can try to assist.
Up-to-date information Please check back often as we will update this page with study information and other relevant FA news and resources. If you have ideas for information that you would like to have covered here please contact us.
What information will participation in the IFAR provide?
At this time our laboratory, headed by Agata Smogorzewska, MD, PhD, is a research laboratory. This means that the information we find cannot be directly released to your family or your doctor. However, we strive to find the genetic cause of FA in every family who participates. If we find the causative FA gene and/or which genetic changes/mutations are present, we will work with you and your doctor to confirm the information so it can be shared with you. The clinical/confirmational testing is ordered by your doctor, will have a cost associated, will require a new DNA sample, but can produce a report that can go in your (your child's) medical chart at your doctor's office and be used by other family members for carrier testing. Having clinical confirmational testing is not a requirement of participation in the IFAR. This is an independent decision and will not affect your ability to participate. This confirmational process of research results applies to all testing performed through our study from July 2009, until the present. Before that time the IFAR maintained a clinical "CLIA-approved laboratory" at The Rockefeller University Hospital, certified by the New York State Department of Health, with Arleen D. Auerbach, PhD, FACMG, as Laboratory Director. This means that findings from most mutation testing done before July, 2009 does not need to be confirmed. Reports from testing before this time can be generated by Dr. Auerbach, upon request through our study coodinator, and must be sent to a health care professional.


Fanconi anemia (FA) is a genetic disease that occurs in approximately 1-2/100,000 live births. FA is a bone marrow failure syndrome. In addition, major physical abnormalities occur in approximately two out of three cases. The types of birth defects and severity of birth defects vary greatly from patient to patient. Some of the more common physical abnormalities include: skin pigmentation (55%), short stature (51%), arms (43%), kidney (21%), ears/hearing (9%), heart and lungs (6%), and digestive system (5%). FA is also a cancer susceptibility syndrome. Individuals with FA have an 800-fold risk to develop AML (or acute myeloid leukemia) and a 500-fold risk to develop solid tumors of the head, neck, genital, and anal regions compared to individuals without FA.
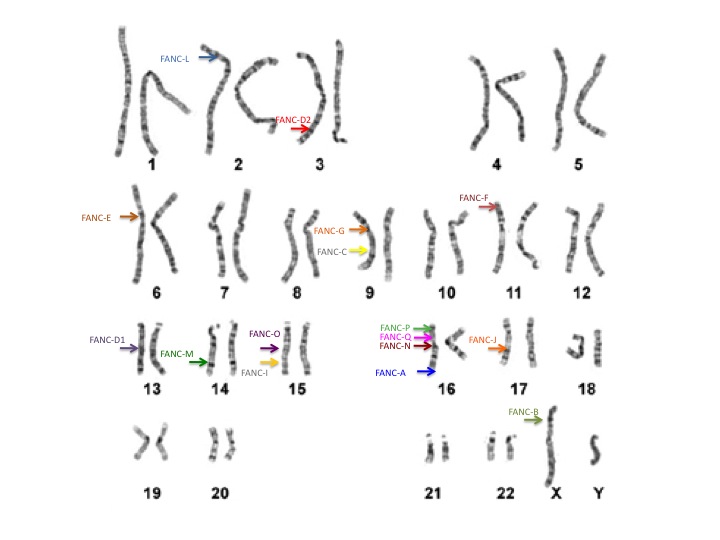
Our genetic information, or DNA, is organized on chromosomes. We each have 46 chromosomes, 23 that come from our mom and 23 from our dad. On each chromosome we have thousands of genes. Because we also receive one gene copy from mom and one from dad, we have 2 copies of each gene. In total, we all have about 25,000 different genes in our bodies. Of these genes, at least 17 published genes are known to be related to FA. These genes are on many different chromosomes.
Everyone has these 17 FA genes in their bodies, but the difference between individuals with and without FA is whether the genes are working properly. When an individual has 2 non-working copies of the same FA gene, he/she is considered to have FA (shown in red below). When an individual has 1 working copy and 1 non-working copy of the same FA gene, he/she is considered to be a carrier (shown in purple below). When an individual has 2 working copies of all of their FA genes, they are unaffected (shown in blue below).
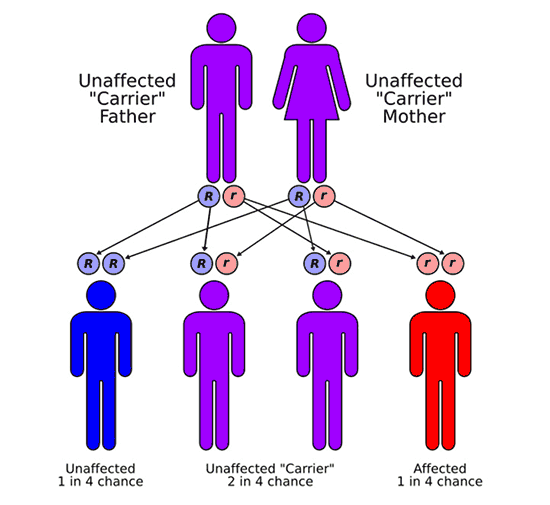 In most cases, FA is a recessive disorder. Meaning that most children with FA have 2 non-working copies of the same FA gene, one that they inherited from their mother and the other from their father. The parents of children with FA are said to be carriers. Most carriers show no signs or symptoms of FA but are at increased risk to have a child with FA. When both parents are carriers of a genetic change or mutation in the same FA gene, they have 3 possible outcomes in each future pregnancy. There is a 25% chance to have an unaffected child; a 50% chance of having a carrier, similar to the parents themselves; and a 25% chance of having a child with FA. With each pregnancy, these risks are the same, regardless of what happened in a previous pregnancy.
In most cases, FA is a recessive disorder. Meaning that most children with FA have 2 non-working copies of the same FA gene, one that they inherited from their mother and the other from their father. The parents of children with FA are said to be carriers. Most carriers show no signs or symptoms of FA but are at increased risk to have a child with FA. When both parents are carriers of a genetic change or mutation in the same FA gene, they have 3 possible outcomes in each future pregnancy. There is a 25% chance to have an unaffected child; a 50% chance of having a carrier, similar to the parents themselves; and a 25% chance of having a child with FA. With each pregnancy, these risks are the same, regardless of what happened in a previous pregnancy.
The FA genes are designated by a letter of the alphabet. The most common gene is FANCA and accounts for approximately 65% of cases. FANCC and FANCG are the two next most common FA genes. FANCB is the only gene known to be inherited in a different way and affects mostly boys. The other FA genes include: FANCD1, FANCD2, FANCE, FANCF FANCI, FANCJ, FANCL, FANCM, FANCN, FANCO, FANCP, FANCQ, and FANCS. 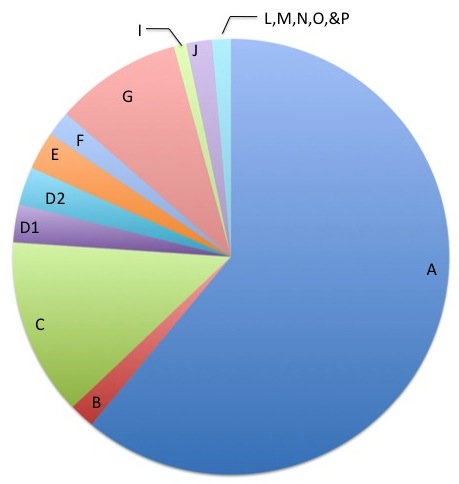
Of these 17 genes, 8 join together in what is called the "core complex". These 8 genes include FANCA, FANCB, FANCC, FANCE, FANCF, FANCG, FANCL, and FANCM. There is also a second grouping among the FANCD2 and FANCI genes, which is called the "ID complex".
When parents are both carriers of different non-working FA genes, there is no increased risk to have a child with FA. However, members of their family, including their children would be at increased risk to be a carrier of FA, and therefore at increased risk to have a child affected by FA. For example, if a woman is a carrier of a mutation in FANCA and her partner is a carrier of a mutation of FANCC (and the child inherited both of these mutations), the child would not have FA. Along these lines, if two individuals with FA conceive a child together, we would only expect the child to have FA if both individuals had the same non-working gene causing their FA.
Testing for Fanconi anemia: The testing for FA can be quite complicated. It usually involves at least 2 different steps:
- Testing to see if an individual has FA or not
This is often called a chromosome breakage test. It can also be called a DEB test or an MMC test. DEB and MMC are chemicals that are added to our genetic structures, called chromosomes. If someone has FA, these chemicals cause the chromosomes to break. This test can only identify if someone has FA or not... it cannot determine which gene is involved. While this test is typically done on a blood sample, sometimes a skin sample is needed. If someone tests positive on this test we say that their cells are "sensitive".
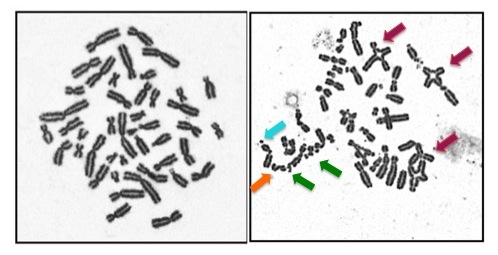
- Testing to determine which FA gene is involved: Complementation testing had been the first step in genetic testing for most families in the past. Now with newer technologies, like Next Generation Sequencing, the step of complementation testing can be bypassed.
- Panel testing/Next generation sequencing is quickly becoming the future of genetic testing since it can give us so much information from a single test. It can be done on DNA from a blood or skin sample. This technology does not require complementation testing first. This testing can be done in different ways. Most often it is used to look at all the FA genes at once, along with other possible genes that could cause FA. Sometimes this technology is used to sequence the entire genetic information at once (called whole exome or whole genome sequencing). This testing produces a lot of data about an individual so it is best to test someone with FA and his/her parents at the same time so we can make sense of the data. This technology is usually followed by specific gene sequencing or other tests to make sure that what was found is causing the FA. This testing is available clinically.
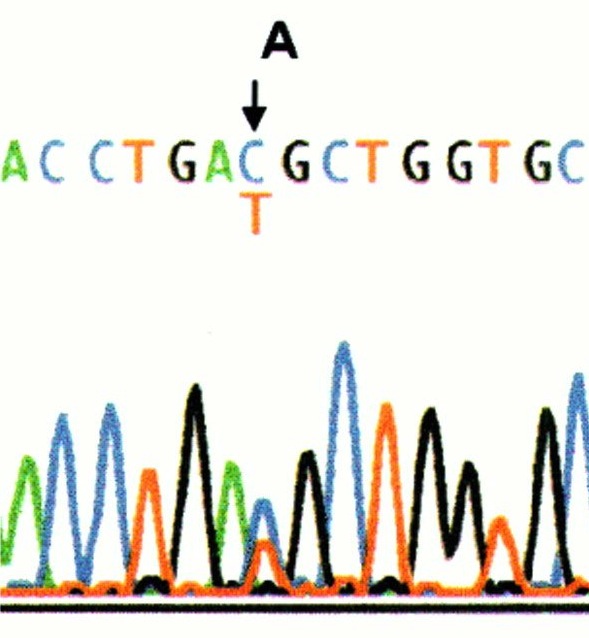
- Specific gene sequencing is often done after complementation testing or if someone is known to have a certain ethnic background. This test spells out each letter of the indicated gene to see if there are any misspellings. Some misspellings are harmless and are called "variants" because they do not cause FA. Other misspellings are called "mutations" meaning that they cause the FA. Sometimes, if a misspelling is found for the first time, we don't know if it is a variant or a mutation and further testing is needed to figure this out.
- Dosage testing (CGH or MLPA) this test is used to see if there are missing or extra bits of genetic information in one of the FA genes, that the sequencing cannot pick up. It is ordered as a separate test in most labs but technologies are rapidly changing to include this analysis in the panel testing. This test is very important if two mutations are not found through sequencing, because many people have deletions that cause their FA.
- Complementation testing tests the proteins involved in FA, as these proteins are made from the FA genes. The testing usually looks at 1 or 3 proteins at a time. It requires a "cell line" which is a source of DNA that can continue to live outside of the body and be studied for many years. First, this test looks to see if the cell line is sensitive (similar to a chromosome breakage test described above). If the cell line is not sensitive, the test cannot be done. If the cell line is sensitive, then the testing can proceed. The idea behind this test is that someone with FA does not produce enough of one of the FA proteins. Therefore, if a normal FA protein is put back into the sensitive cell line, then the cell line should no longer be sensitive. In other words, adding the normal protein corrects for the missing protein. This test can tell us which protein is missing and therefore assign the patient into one of the groups, which in turn tells us which gene is not working. These groups are often referred to as the "complementation groups". They include: FA-A, FA-B, FA-C, FA-D1, FA-D2, FA-E, FA-F, FA-G, FA-I, FA-J, FA-L, FA-M, FA-N, FA-O, FA-P, and FA-Q. This test cannot find the specific genetic changes (mutations) in that gene however.
Each test described above has benefits and limitations. We have summarized some of those benefits and limitations in the table below. The time it takes to get results and the associated costs are specific to each laboratory and are always changing. This information is to give you a general estimate but actual time and cost may be higher or lower when the test is ordered. It is important that the right test be ordered to ensure proper diagnosis and medical management. This testing can be very confusing to doctors and families alike. We are always happy to speak with your/your child's doctor to help navigate this process if it would be beneficial.
| Test | Purpose of test |
Approx time to get results |
Approx Cost | Cons/Limitations |
| Panel testing/ Next generation sequencing |
-Find variations/mutations in any FA gene |
6-10 Weeks | $2-5,000 |
-Cost |
| Targeted mutation analysis | Determine if a known mutation is present or absent | 1-3 Weeks | $2-450 per mutation | -Only helpful if the mutation is known in the family or the family is of a specific ethnic background |
| Single gene sequencing | Find variations/mutations in any single FA gene | 2-4 Weeks | $400- $1800 per gene | -Can be costly and take a lot of time if complementation testing is not done first -Cannot find deletions or duplications |
| Dosage testing (CGH/MLPA) | Find any deletions (missing DNA) or duplications (extra DNA) in any FA gene | 3-4 Weeks | $800- $1500 total | -Cannot find misspellings in the DNA |
| Complementation | Identify which gene is causing the FA | 6 Months | $2500 for 3 genes | -Requires cell line -May require skin biopsy -May not work for all patients |
Within each category, testing can be either clinical or research. You can pursue both research testing and clinical testing at the same time.
Clinical testing: This refers to testing that is performed in a "CLIA-approved" laboratory. CLIA laboratories follow very specific laws and regulations. By law, these clinical laboratories can produce a report with the results of the testing. The primary goal in clinical testing is to gain information for the patient/family. The benefits of clinical testing are that it is done much faster (and you can know how long it will take before the test is ordered) and it produces a report that can be shared with physicians and family members. The limitation of clinical testing is that it can often be expensive and only a few labs may do the desired test.
Research testing: By law, results from research testing cannot be shared with the patient/family and sometimes even the doctor. If you would like results from research testing, it has to first be confirmed in a clinical lab. The primary goal in research testing is to learn more from a science standpoint and not necessarily to benefit the patient/family directly. So why does anyone do research testing? Sometimes, a test is only available in a research setting. Other times, it saves money as all of the research testing is done for free and helps make the clinical testing more meaningful or informative. Finally, the hope is that the research will help us understand FA better so that we can help the FA community as a whole in the future. The downside of research testing is that it can take a long time and we cannot predict how long it might take. Here, at The Rockefeller University, we currently do research testing only.
FOR INDIVIDUALS ALREADY ENROLLED IN THE IFAR
Thank you for your participation in the IFAR. We could not do this study without you! But we still need your help!!! Currently, we have approximately 1278 families involved in the IFAR, which is wonderful. However, it makes keeping track of the most up-to-date information about each family difficult. The research only works if the communication is two ways. We want to help you, and we also need your help.

Here are ways you could help us:
- If you/your child has a doctor’s appointment or has blood counts done please remind the physician of your participation in the IFAR, and ask that a copy of the labs/clinic note be sent to us at: Dr. Agata Smogorzewska, The Rockefeller University 1230 York Avenue, Box 182, New York NY, 10065, or faxed to 212-327-8262.
- If you have not spoken directly to us, we would love to hear from you in the way of general introductions, so we can get to know your family better. We are always interested in hearing how you/your child is doing. We appreciate any updates you can provide us along the way.
- If you have a child who is now 18, we need his/her permission to be able to continue his/her participation in the research. We are slowly going through our files to try to identify these participants who are now over 18, but if you know your family is in this situation please contact us at fanconiregistry@rockefeller.edu or 212-327-8612 to review the paperwork needing to be updated.
- Check back to this website or our IFAR facebook page regularly to learn of new study opportunities within the IFAR.
How to contact/update us:
![]() FACEBOOK: Please "friend" us as our IFAR Facebook account allows for regular communication with families
FACEBOOK: Please "friend" us as our IFAR Facebook account allows for regular communication with families
![]() EMAIL: Study coordinator, fanconiregistry@rockefeller.edu
EMAIL: Study coordinator, fanconiregistry@rockefeller.edu
![]() PHONE: Study coordinator: 212-327-8608
PHONE: Study coordinator: 212-327-8608
For participating families who have not yet received notification that we have research results available, we greatly appreciate your patience. We know this is a very long and often frustrating wait. If at any point you are looking for an update or want to check with us to see if any results are available, please do not hesitate to contact us. Please remember if we have results that were reported after July 2009, we will not be able to give you the details of the findings until they are confirmed in a clinical laboratory. However, we will update you about the status and next steps.
FOR INDIVIDUALS NOT YET ENROLLED IN THE IFAR:

Who can participate in the IFAR? As the name suggests, we are an international registry. As such, any individual who has been diagnosed with FA, or has features similar to FA is able to participate. In addition, we like to enroll family members of that individual when possible, as we know genetic testing is most informative when an affected individual and his/her parents are studied at the same time.
What is involved in participation in the IFAR? For all individuals with FA (or FA-like symptoms) in the IFAR we collect medical information and often a DNA sample (DNA is the genetic information in our bodies). The medical information we collect is from medical professionals and family members, where appropriate. If the genetic cause of FA is already known in your family, then we would not need a DNA sample in most cases. When we do need a DNA sample, it is usually obtained through a blood draw. However, if a person has already had a bone marrow transplant, or diagnostic testing for FA has showed ambiguous results, we often try to arrange a skin biopsy as well. From the DNA sample we create a cell line, which is a group of cells that can live and grow outside of the body. They can be frozen and can be used for future research. For family members of someone with FA or FA-like symptoms, we gather relevant medical history and also try to obtain a blood sample to have DNA for the testing.
What are the benefits of participating in the IFAR?
- There may be no direct benefit from your participation in this study
- The benefits may be indirect. Our improved understanding of FA may lead to changes in diagnosis and management which could potentially benefit you/your child
- We may be able to find the genetic cause of the FA in your family, which might not only benefit you/your child, but other family members as well
What are the risks of participating in the IFAR?
- Discomfort associated with a blood draw
- Discomfort and possible scarring associated with a skin biopsy
- Theoretical risk of loss of privacy if significant amounts of genetic sequencing are performed
If I/my family is not already enrolled in the IFAR how do we get involved?
- You can always contact our genetic counselor/study coordinator at the Fanconi registry at 212-327-8612 or fanconiregistry@rockefeller.edu at any time.
- If you/your child is followed by one of the FA Comprehensive Care Centers (Memorial Sloan Kettering Cancer Center, University of Minnesota, Cincinnati Children's Hospital Medical Center) you can speak to your coordinator or physician there as all three centers are part of our study.
- If you do not feel comfortable contacting our study coordinator directly, you can ask your/your child's doctor to contact us.
FORMS YOU MAY NEED FOR IFAR PARTICIPATION:
- Consent form Required for every individual who participates in the IFAR, with or without FA. It details all of the risks, benefits, and limitations of the study
- Minors - either parent can check one of the options on page 7 and both parents (where possible) should sign and date on page 9.
- Adults - please check one of the options on page 7, sign and date at the top of page 8 (parents of a child with FA, should sign and date this form with their own name, and not the child's)
- Assent form I For individuals ages 7-18 with FA
- Assent form II For individuals ages 7-18 without FA
- HIPAA form Health Insurance Portability and Accountability Act - required for every individual who participates in the IFAR, whether they have FA or not.
- Minors - only one parent needs to sign. On page 3, the parent should add his/her own name on the top left line and the child's name under the name of the participant. The parent should print his/her own name under "printed name of legal representative", indicate his/her relationship to the child (ex. mother or father) and date the form.
- Adults - you are considered the participant on this form, even if you are an unaffected parent. Please sign and print your name on the left hand side of page 3 and date on the right. Everything else can be left blank.
- Information sheet This form documents contact information for our participants and their physicians. It helps us stay in touch, especially in case of a change of address.
- Release form for annual records The IFAR aims to collect data about FA over the course of a person's life. This is called longitudinal data. Ideally your/your child's doctor would send us a copy of the note from your recent visit and laboratory studies. However, doctors are usually very busy and often not able to do this. As such, we request records on an annual basis to stay updated about your/your child's health. This forms allows us to do that annually without getting a signature each year. You can decide to withdraw this form/permission at any time by calling or emailing one of our study coordinators. If you agree to allow us to request annual records please use the following instructions:
- Put your/your child's name on the top line.
- Put your/your child's hematologist name and information on the middle line.
- Sign and date the appropriate line at the bottom.
- Release form to release clinical results If you were tested in our study before July 2009, your/your child's results may be clinical. This means that a report can be generated from the testing and released to a health care professional. If you would like us to release clinical results please:
- Add the health care professional's name that you would like us to release the results to at the top.
- Please put your/your child's name on the "Participant tested" line indicating the person's results to be released.
- Sign and date on the applicable line below.
- Release form to release research results Research results must be confirmed in a clinical laboratory before they can be released. This form gives us permission to notify your/your child's medical professional, as we will need his/her help in this process.
- If you have a genetic counselor or geneticist who works with your family, please put his/her name where indicated. If not, please use your/your child's hematologist.
- We also need your permission to release these results to the laboratory doing the testing so they know exactly which test to do. You may have the choice of which lab to use or it may be determined by the hospital's contract with the laboratories.
- On the "Participant Tested" line please put your own name if you have FA, or your child's name if you are a parent of an individual with FA.
- There are three signature/date lines at the bottom and only one of the three needs to be signed. If you are a parent signing for a minor, please use the top option. If you are an adult signing for yourself please use the middle line.
- Medical update form
History of the IFAR: 
The IFAR was founded in May 1982, by Dr. Arleen D. Auerbach, in collaboration initially with Dr. Traute Schroeder-Kurth of Germany. Schroeder-Kurth, in 1964, discovered the chromosome instability in Fanconi anemia patients and Auerbach, in 1981, developed the DEB (diepoxybutane) chromosome breakage test. From May 1982 until closing in December 2007, Auerbach’s Cytogenetics Laboratory at The Rockefeller Hospital was New York State licensed and CLIA-certified. The laboratory issued diagnostic reports on many patients from North America with clinical findings resembling FA. Patients with a positive DEB test were eligible to participate in the IFAR study, resulting in the enrollment of a large number of patients with FA. Studies of IFAR patients led to the discovery that the clinical features of the syndrome were much more varied than originally thought, and to the recognition of the need for a more timely diagnosis of the disease. The IFAR Tissue Repository of specimens from patients with a positive DEB test aided researchers over the years to identify new genes for FA, and to find a large spectrum of changes in these genes that cause the disease (click here for IFAR publications). In 2011, Agata Smogorzewska, M.D., Ph.D. Head of the Laboratory of Genome Maintenance at the Rockefeller University became the Principal Investigator of the IFAR, with Auerbach remaining as an Investigator on the IFAR protocol and Consultant to the Laboratory of Genome Maintenance.
- Fanconi Anemia Comprehensive Care Centers
- Director: Dr. Stella Davies
- Coordinators: Erica Goodridge, Michelle Harris, Kathleen Ball
- Phone: 513-636-3218
- Director: Dr. John Wagner
- Coordinator: Rebecca Tryon, MS, CGC
- Phone: 612-273-2800
- Director: Dr. Farid Boulad
- Phone: 212-639-6684
- Cincinnati Children's Hospital Medical Center
- University of Minnesota Medical Center and Amplatz Children's Hospital
- Memorial Sloan Kettering Cancer Center (New York)
- Fanconi Anemia Research Fund
- The Other Kid. A child friendly workbook designed to help children cope with the emotional impact of having a brother or sister with a serious medical condition

PLEASE COME BACK AND VISIT OUR WEBSITE AGAIN SOON
If you have suggestions for what would be helpful to you on our website, please email Fanconi registry
If you are willing to have your/your child's picture appear on our website, please email Fanconi registry

