Research
Anti-inflammatory activity of IVIG/2,6-sialylated Fc is medicated by SIGNR1 and FcgRIIB
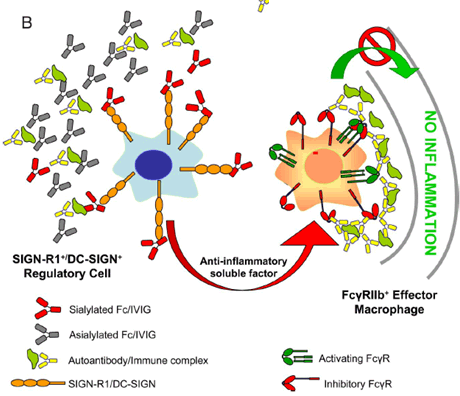
The anti-inflammatory activity of intravenous Ig (IVIG) results from a minor population of the pooled IgG molecules that contains terminal alpha2,6-sialic acid linkages on their Fc-linked glycans. These anti-inflammatory properties can be recapitulated with a fully recombinant preparation of appropriately sialylated IgG Fc fragments. These sialylated Fcs require a specific C-type lectin, SIGN-R1, (specific ICAM-3 grabbing non-integrin-related 1) expressed on a regulatory macrophage populaton in the splenic marginal zone. The consequence of this interaction is the development of an anti-inflammatory response, presumable mediated through a soluble factor, that acts on effector macrophages to enhance expression of the inhibitory FcRIIB molecule. Upregulation of FcRIIB alters the threshold required for immune complex triggered inflammation by these effector cells and results in an anti-inflammatory response. The human orthologue of SIGN-R1, DC-SIGN, displays a similar binding specificity to SIGN-R1 but differs in its cellular distribution, potentially accounting for some of the species differences observed in IVIG protection.