Research
IgG Rc Receptors
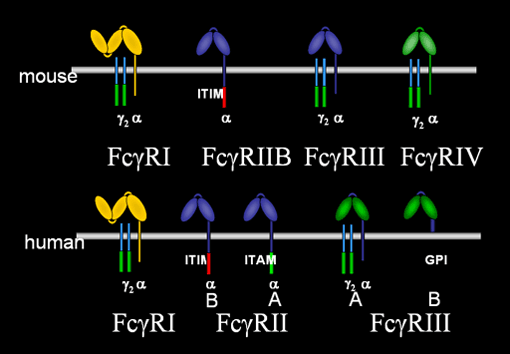
The first cloning of Fc receptors was reported by the Ravetch lab in 1986 and subsequent studies have revealed a multi-gene family of oligomeric receptors for the Fc domain of antibodies. Mice deficient in specific FcRs were generated by that group in 1994 and has lead to a detailed understanding of the role of these receptors in inflammation and modulation of the immune response. FcRs can either activate cellular responses through ITAM mediated mechanisms or inhibit those responses through ITIM sequences.

The structure of the complex formed by the extracelular domain of FcRIII and human IgG1 reveals the critcal role of the CH2 domain and its associated biantennary glycan.
Sondermann, et al. 2000
Fc receptors for IgG are now recognized as forming the essential link between antigen recognition, inflammation and immune modulation. IgG antibodies cytotoxic for target cells, such as pathogenic microorganisms or tumor cells require the presence of FcRs to mediate their biological activities. Immune complex triggered inflammation, a hallmark of autoimmune diseases like lupus, are similarly dependent on Fc receptor engagement and independent of the classical pathway of complement activation. FcRs are potent regulators of the immune response, contributing to the maintenance of peripheral tolerance by regulating dendritic cell activation and plasma cell survival. Modulation of FcR responses is central to the efficacy of IgG antibodies, to the development of autoimmunity and regulation of the immune response. Programs to exploit these pathways are underway to develop improved antibody therapeutics, vaccination strategies and treatments for autoimmune diseases.
