Xiao Tao
B.S. Beijing University
Ph.D. Columbia University

Potassium channels represent the most widely distributed and functionally diverse family of ion channels. They are involved in most important physiological processes of the cells. Based on their topologies, there are four major subfamilies of potassium channels: i). Voltage-gated potassium channels (Kv); ii). Inward-rectifier potassium channels (Kir); iii). Ca++-activated potassium channels; and iv). Tandom-pore-domain potassium channels (K2P). I have studied the voltage-gated as well as the inward-rectifier potassium channels by a combination of X-ray crystallography and electrophysiology, mainly interested in understanding their differential gating mechanisms.
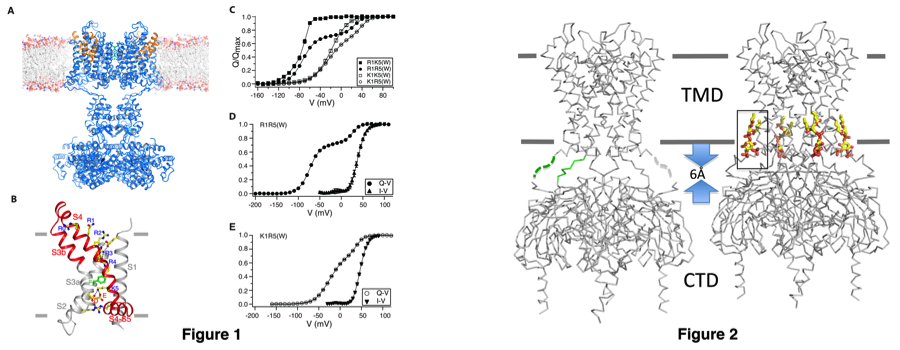
Kv channels are responsible for the re-polarization of action potential in neurons and muscle. They are composed of a central K+ conduction pore surrounded by four voltage sensors. The voltage sensor domain reads the membrane voltage through the gating charges resided on its fourth transmembrane helix (S4) and undergoes conformational changes that are coupled to the open/close of the pore. The question I have been trying to address is how does the voltage sensor move in the membrane in response to membrane voltage change, and how does this movement gate the pore open and close? By determining a 2.4Å X-ray structure of a chimeric Kv channel (paddle-chimera channel), in which the voltage sensor paddle has been transferred from Kv2.1 to Kv1.2, we have revealed the molecular details of the depolarized voltage sensor and open pore embedded in a lipid-membrane like environment (Fig. 1A)(ref 1). The structure helped explain how the gating charges are stabilized in the low-dielectric membrane through interacting with the internal and external negative charge clusters. Further studies using mutations with natural and unnatural amino acids, electrophysiology and x-ray crystallography allowed us to identify a gating charge transfer center in voltage sensors that facilitate the gating charge movement (Fig. 1B)(ref 2). The gating charge transfer center is formed by a rigid cyclic "cap" on the top and two conserved acetic residues at the bottom and specific mutations of the cap favors lysine relative to arginine. This preference allowed us dissect the voltage sensor movements and the coupling to the channel opening by putting lysine at different locations (Fig. 1C-E). Capture of different conformations in the crystal structures is currently under effort to further understand the gating mechanism.
Kir channels featuring a large cytoplasmic pore and PIP2 activation conduct K+ ions most efficiently into the cell. Among them, the classical strong inward-rectifier Kir2 channels control the resting membrane potential in many electrically excitable cells and heritable mutations cause periodic paralysis and cardiac arrhythmia. I have determined the crystal structure of Kir2.2 in the absence of PIP2 that revealed the structural basis for understanding rectification (Fig. 2)(ref 3). Further determination of the channel-PIP2 complex structure provided the structural basis of PIP2 activation and allowed us to propose a potential activation mechanism by PIP2 (Fig. 2)(ref 4). Mechanistic studies on PIP2 activation is currently undergoing to further understand the molecular mechanism of PIP2 activation of Kir channels.
References:
- Stephen B. Long, Xiao Tao, Ernest B Campbell, Roderick MacKinnon. (2007) Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature 450(7168), 376-82.
- Xiao Tao, Alice Lee, Walrati Limapichat, Dennis A. Dougherty and Roderick MacKinnon. (2010) A gating charge transfer center in voltage sensors. Science 328(5974), 67-73
- Xiao Tao, Jose L. Avalos, Jiayun Chen and Roderick MacKinnon. (2009) Crystal structure of the eukaryotic strong inward-rectifier K+ channel Kir2.2 at 3.1 Å resolution. Science 326(5960), 1668-74.
- Scott B. Hansen, Xiao Tao and Roderick MacKinnon. (2011) Structural basis of PIP2 activation of the classical inward rectifier K+ channel Kir2.2. Nature 477(7365), 495-8
