Ernest Campbell
B.S. – Cornell University (1975)

The research goal is to obtain three-dimensional structures of cation and anion integral membrane channels. To this end, I have worked with a number of postdoctoral associates in the laboratory on elucidating the structures and functional implications of the prokaryotic ClC, the eukaryotic voltage-gated potassium channel Kv1.2 with its beta subunit and a eukaryotic CmClC with its intracellular domain. We have worked extensively on the development and screening of monoclonal antibody fragments for use in crystallization of various ion channels such as the E.coli ClC, KvAP, TRAAK and Kcsa potassium channels, as well as the isolated voltage-sensor of KvAP.
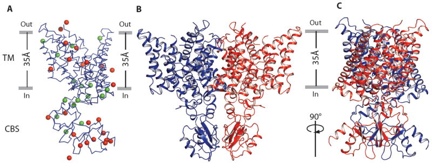
Structure of CmCLC
Selected Publications:
Feng L, Campbell EB, MacKinnon R. (2012) Molecular mechanism of proton transport in CLC Cl-/H+ exchange transporters. Proc Natl Acad Sci U S A. 109(29), 11699-704
Feng L, Campbell EB, Hsiung Y, MacKinnon R. ( 2010) Structure of a eukaryotic CLC transporter defines an intermediate state in the transport cycle. Science, 330(6004), 635-41
Long, S.B., Tao, X., Campbell, E.B. & MacKinnon, R. (2007). Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature, 450, 376-82. PMID: 18004376.
Long, S.B., Campbell, E.B. & Mackinnon, R. (2005). Voltage sensor of Kv1.2: structural basis of electromechanical coupling. Science, 309(5736), 903-8.
Dutzler, R., Campbell, E.B. & MacKinnon, R. (2003). Gating the selectivity filter in ClC chloride channels. Science, 300(5616), 108-12.
Dutzler, R., Campbell, E.B., Cadene, M., Chait, B.T. & MacKinnon, R. (2002). X-ray structure of a ClC chloride channel at 3.0 A reveals the molecular basis of anion selectivity. Nature, 415(6869), 287-94.
Shrivastava, A., Radziejewski, C., Campbell, E.B., Kovac, L, McGlynn, M., Ryan, T.E., Davis, S., Goldfarb, M.P., Glass, D.J., Lemke, G. & Yancopoulos G.D. (1997). A orphan receptor tyrosine kinase family whose members serve as non-integrin collagen receptors. Molecular Cell, 1(1), 25-34.
Moy, F.J., Seddon, A.P., Campbell, E.B., Böhlen, P. & Powers, R. (1995). 1H, 15N, 13C and 13CO assignments and secondary structure determination of basic fibroblast growth factor using 3D heteronuclear NMR spectroscopy. J. Biomol. NMR., 6(3), 245-54.
Hattori, Y., Campbell, E.B. & Gross, S.S. (1994). Argininosuccinate synthetase mRNA and activity are induced by immunostimulants in vascular smooth muscle. Role in the regeneration of arginine for nitric oxide synthesis. J. Biol. Chem., 269(13), 9405-8.
Campbell, E.B., Hayward, M.L. & Griffith, O.W. (1991) Analytical and preparative separation of the diastereomers of L-buthionine (SR)-sulfoximine, a potent inhibitor of glutathione biosynthesis. Anal. Biochem., 194(2), 268-77.
Campbell, E.B. & Griffith, O.W. (1989) Glutathione monoethyl ester: high-performance liquid chromatographic analysis and direct preparation of the free base form. Anal. Biochem., 183(1), 21-5.
Hayward, M.A., Campbell, E.B. & Griffith, O.W. (1987). Sulfonic acids: L-homocysteinesulfonic acid. Methods Enzymol., 143, 279-81.
Griffith, O.W. & Campbell, E.B. (1987). Resolution of cysteine and methionine enantiomers. Methods Enzymol. 143, 166-72.
Griffith, O.W., Campbell, E.B., Pirkle, W.H., Tsipouras, A. & Hyun, M.H. (1986). Liquid chromatographic separation of enantiomers of beta-amino acids using a chiral stationary phase. J. Chromatogr., 362(3), 345-52.
