Laboratory of Molecular Genetics
Jeffrey M. Friedman
Professor; Investigator, HHMI
The application of modern methods in genetics has led to the identification of a new hormone, leptin, that regulates body weight. Leptin is an adipose tissue hormone that interacts with receptors in the brain to regulate food intake, energy expenditure and other neuroendocrine systems. The molecular mechanisms of leptin in the brain are under investigation. These studies are being conducted in parallel with efforts to identify obesity genes in the human.
Research Projects:
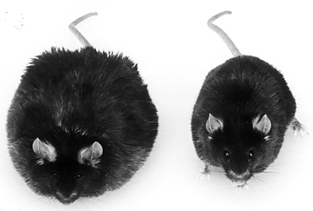
© 1995 Amgen Inc.
Cholecystokinin:
Although the physiological regulation of body weight and appetite has been strongly suggested by experimental evidence, the elucidation of the relevant molecular mechanisms has proven difficult. The possible role of a brain-gut peptide, cholecystokinin (CCK), in these processes was the initial subject of investigation in this laboratory. CCK has been extensively evaluated as a possible satiety factor. CCK is secreted as a 33 amino acid peptide from endocrine cells in the jejeunum where it is released in response to nutrient in the intestinal lumen. The same CCK precursor is posttranslationally processed to an 8 amino acid peptide in brain. The single copy CCK gene is differentially regulated in brain and intestine during development and expressed ectopically in a class of primitive neuronal tumors3-6. The physiological role of CCK in controlling appetite is unclear. In 1973 Smith and Gibbs showed that injections of CCK reduce food intake in food deprived rodents. In addition, the levels of brain CCK were reported by Straus et al to be low in genetically obese (ob) mice8. However, nonpeptide CCK antagonists developed by Squibb and other pharmaceutical companies do not affect food intake and body weight in the long term9. Moreover, overexpression of CCK in transgenic mice did not affect food intake or body weight (unpublished data). Genetic mapping of the CCK gene to mouse chromosome 9 excluded it as being etiologic in any of the inherited rodent obesity syndromes10. These data raised the question as to the molecular basis of the phenotype in genetically obese (ob) and diabetic (db) mice.
Molecular Cloning of Leptin and its Receptors
Mutations in the mouse ob and db genes result in obesity and diabetes in a syndrome resembling morbid human obesity11, 12. Coleman, using the method of parabiosis, predicted that the ob gene encoded a novel hormone and that the db gene encoded its receptor11. Recent data from this laboratory are consistent with this hypothesis. The ob gene was identified by positional cloning and found to encode a 4.5 kB RNA expressed exclusively in adipocytes13-16. The ob gene product, known as LEPTIN, circulates as a 16 kilodalton protein in mouse and human plasma but is undetectable in plasma from C57BL/6J ob/ob mice17. Plasma levels of this protein are increased in diabetic (db ) mice, a mutant thought to be resistant to the effects of ob17. The levels of protein are also increased in several other genetic and environmentally induced forms of rodent obesity including mice with lesions in the hypothalamus16. Daily intraperitoneal injections of recombinant mouse leptin reduced body weight of ob/ob mice by 30% at 2 weeks and by 40 % after four weeks but had no effect on db/db mice17. The protein reduced food intake and increased energy expenditure in ob/ob mice. Injections of wild type mice twice daily with the mouse protein resulted in a sustained 12% weight loss, decreased food intake and a reduction of body fat from 12.2 to 0.7%. Recombinant human leptin reduced body weight with equivalent potency to mouse leptin when injected into ob mice17. In human, the plasma level of leptin correlated with body mass index (BMI) and % body fat18. However at a given BMI, there was significant variability in the leptin level. In all cases analyzed weight loss in human was associated with a decrease in plasma leptin concentration18. These data suggest that leptin serves an endocrine function to regulate body fat stores. In most instances, obesity is associated with an apparent decrease in sensitivity to endogenous leptin resulting in a compensatory increase in adipocyte mass. However, in a subset of cases human obesity appears to result from subnormal leptin secretion18-20.
The complete insensitivity of db mice to leptin and the identical phenotype of ob and db mice suggested that the db locus encodes the leptin receptor 11, 17. The db gene was localized to a 300 kB interval on mouse chromosome 419-21. Exon trapping and cDNA selection identified a candidate gene in this region. This candidate was found to be identical to a receptor (ob-R) which was functionally cloned from choroid plexus21, 22. However, because this receptor was normal in db mice, the possibility was raised that the db mutation affected an alternatively spliced form. The Ob-R gene was found to encode at least five alternatively spliced forms 21. One of the splice variants is expressed at a high level in the hypothalamus and at a lower level in other tissues. This transcript is mutant in C57BL/Ks db/db mice21. The mutation is the result of abnormal splicing leading to a 106 bp insertion into the 3' end of its RNA. The mutant protein is missing the cytoplasmic region and is likely to be defective in signal transduction. A nonsense mutation in facp rats, a rat equivalent of db, leads to premature termination NH2-terminal of the transmembrane domain (unpublished data). These data suggest that the weight reducing effects of leptin are mediated by signal transduction through a receptor in the hypothalamus and elsewhere.
Further studies have revealed that the Stat3 transcription factor is activated specifically in hypothalamus within 15 minutes of a single injection of leptin in ob and wild type but not in db mice23. In situ hybridization indicates that Ob-Rb is expressed in three different hypothalamic regions: the arcuate, ventromedial and lateral hypothalamic nuclei (in preparation). Lesions of each of these nuclei are known to affect body weight regulation. Further characterization of the neurons in these brain regions and their connections will have important implications for our understanding of leptin's actions and the molecular mechanisms regulating body weight.
Genetic Basis of Obesity in Humans
Advances in genetics make it possible to identify human disease genes. The implementation of a genetic approach to the study of obesity will help establish whether the human ob or db genes account for genetic forms of obesity and also lead to the identification or validation of other candidate genes. Such studies require that large numbers of families be collected in which the trait of interest is inherited.
In order to implement this approach for the study of obesity, this laboratory has developed a collaboration with the Department of Health on the island of Kosrae in Micronesia. The citizens of this island have a high incidence of obesity, the basis of which is not understood. The Kosraen population is highly admixed between Micronesian and Caucasian ancestors, a fact that facilitates genetic analysis. A study has now been completed in which the entire adult population of Kosrae over twenty years of age, ~2500 individuals, has had a complete medical workup including measurements of height, weight, blood pressure, and glucose levels. In addition, measurements of serum insulin, and eventually leptin, will be made. Measurements of serum cholesterol, and triglycerides have already been completed by Dr. Jan Breslows laboratory at Rockefeller University. In collaboration with the Stoffel laboratory, DNA has been isolated from each individual as well as information about the identity and medical status of other family members. To date, all 2500 DNA samples have been processed ad genetic analyses have begun. The availability of a complete clinical profile on an entire population, combined with modern methods in genetics should make it possible to establish the possible relationship of genetic variation at the human ob and db genes to human obesity. In addition, a highly admixed population provides an opportunity to identify additional loci that affect the control of body weight, as well as the medical problems that are often associated with obesity such as hypertension, diabetes, heart disease.
Future Plans:
Future studies will also focus on the physiologic effects of leptin. These include studies of leptin's effects on lipid metabolism, glucose metabolism and insulin action. Available data suggest that neurons in the hypothalamus are a principle target of leptin actin. Studies to establish the neurotransmitter profile and projection of Ob-Rb positive neurons have begun. Analysis of the electrophysiologic effects of leptin on these cells will proceed simultaneously. Efforts to produce a higher activity version of leptin are also underway in studies of the structure function relationship of leptin and its receptors (collaborative with the Burley laboratory).
