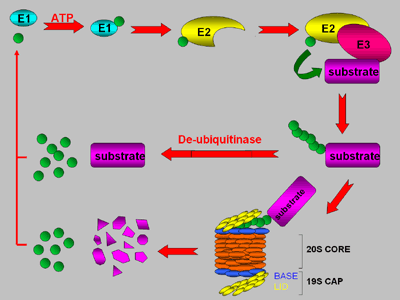

The ubiquitin/26S proteasome pathway. Following cleavage of ubiquitin precursors to expose the C-terminal Gly-Gly residues,ubiquitin is activated by an E1 to form a thiol ester and then transferred to an E2. An E3 recruits the substrate to the E2 to facilitate serial attachment of ubiquitin monomers to a Lys residue(s) in the substrate. The conjugate is either recognized by the 26S proteasome for degradation of the substrate or disassembled by a dedicated protease to release the intact protein and ubiquitin monomers. Ubiquitins released by de-ubiquitination can be recycled for further rounds of ubiquitination.