Laboratory of Neurobiology and Behavior
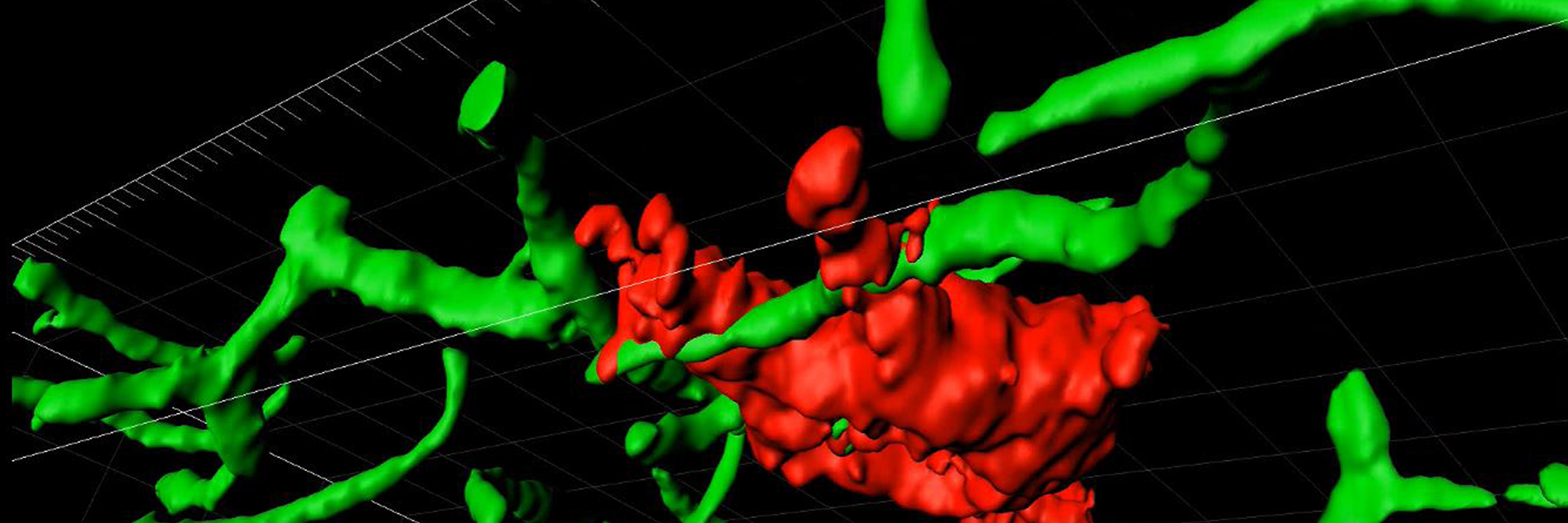
The Pfaff lab, which investigates the cellular control mechanisms for behavior, has two areas of focus: steroid hormones’ influence on the brain and generalized arousal of the central nervous system. Over several years, they solved the question of how hormone action in the brain regulates a specific social behavior (see Lab History).
Current work in Pfaff’s lab focuses on the question: How do animals or humans initiate any behavior at all? His work began to answer the question by framing the concept of generalized arousal (GA, see Books). Pfaff offered the first operational definition of GA, which activates all behavioral responses, enabling scientists to measure arousal quantitatively. In humans, deficits in GA contribute to a wide variety of cognitive and emotional problems, from depression to dementia.
Pfaff and physicist Jayanth Banavar have gathered large amounts of precise behavioral data to test their theory that rapid alterations between not/active and active/aroused act like a physical “phase transition.” The lab measured the behavior of individual mice in GA assay chambers (see Assay) at 20-millisecond resolution and described the behavioral changes precisely in mathematical terms (see Equations). On a longer, circadian time scale, the transition from light (not aroused) to dark (aroused) follows an entirely different equation precisely—and from dark back to light shows a surprising reverse symmetry.